The principle of gold extraction by mixed mercury method
It is a simple and old method to extract gold by mixing mercury. It is based on the principle that gold particles are easy to be selectively wetted by mercury, and then mercury diffuses into gold particles to form gold amalgam (containing amalgam). The mixed mercury reaction can be expressed as follows:
Au +2Hg = AuHg2
The composition of gold amalgam (paste) varies with its gold content. In the process of mixing mercury, the surface of gold particles is first wetted by mercury, and then mercury diffuses into gold particles to form AuHg2、AuHg、Au3Hg and finally the solid solution of gold in mercury is formed. When the mercury paste is heated above 375 ℃, the mercury volatilizes in the form of elemental mercury and gold exists in the form of sponge gold.

Generally, mercury blending is not an independent process, but a combined process with other separation methods. In most cases, mercury blending is only an auxiliary method to recover gold. Due to the poor working conditions and high labor intensity of mercury operation, it is easy to cause mercury poisoning, and the waste gas and wastewater containing mercury should be purified. At present, it is gradually replaced by flotation or gravity separation. However, the mixed mercury method can recover the monomer natural gold, and can be used for local gold, so it still occupies a certain position in gold beneficiation.
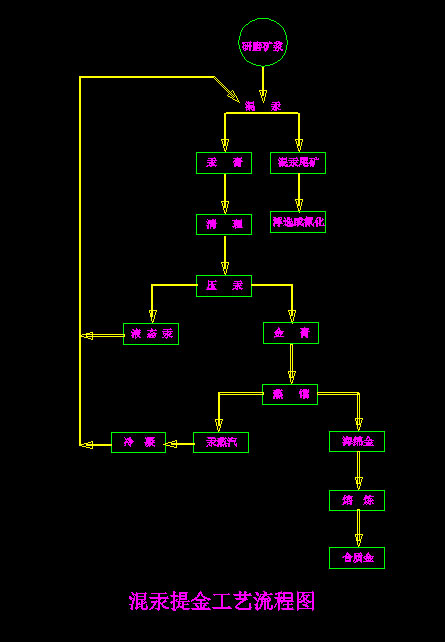
The process flow of extracting gold from mixed mercury is as follows:
What are the factors that affect the recovery rate of mixed mercury extraction?
The recovery rate of gold depends on the particle size and shape of natural gold, fineness of gold particle, quality of mercury, mixing temperature, pulp concentration, mixing method, slope of mercury plate and equipment
1. Influence of raw ore properties on mercury mixing operation
The shape of gold particle size and the degree of monomer dissociation are mainly related to the crushing and grinding operation, especially the degree of monomer dissociation. Properly increasing the grinding fineness can improve the recovery rate of mercury mixing operation. The gold particle suitable for mercury mixing is generally 0.2-0.3 mm, and the lower limit of the particle size of mercury mixing plate in the grinding cycle is 0.015 mm, but the fine gold particles are lost with the pulp.
2.The influence of key parameters on recovery in gold separation process
(1) Ways of mixing mercury and pulp concentration
The fineness of placer gold is higher than that of vein gold, the fineness of gold in oxidation zone is higher than that of primary ore, and the gold with high fineness is easy to mix mercury. The mixed mercury can be divided into internal mixed mercury and external mixed mercury. The external mixed mercury is a process of extracting gold by mixing mercury outside the crushing mill, and the fixed mixed mercury plate and vibrating mixed mercury plate are commonly used in domestic; Internal mixing of mercury is a process of extracting gold by mixing mercury in the crushing and grinding operation cycle. Gold mines in South Africa and the United States often mix mercury in the rammer, small and medium-sized gold mines in the Soviet Union often use the rolling mill, and mercury mixing cylinder is often used in China. The efficiency of internal mixing mercury is higher than that of external mixing mercury, and the quality of mercury and gold is better. The concentration of the external mixed mercury slurry should not be too high to form a loose thin slurry flow, and the flow velocity should not be too high, so that the gold particles can settle on the mercury plate. The suitable concentration of internal mixed mercury slurry is 30-50%, and the mercury should be suspended.
(2) PH of pulp
The pH of pulp has a great influence on the effect of mercury mixing. It is better to mix mercury in acid medium and cyanide solution, but when there are many slimes, the slime can't agglomerate in acid medium, which pollutes the surface of gold particles and affects the effect of mixing mercury. When mixing mercury in alkaline medium, such as using lime as regulator to precipitate soluble salt and eliminate the adverse effect of oil quality, the effect is better when pH = 8-8.5.
(3) Quality of mercury
The quality of mercury has a great influence on the effect of mixed mercury, but the wetting effect of pure mercury on gold is not good. A small amount of gold, silver and base metals in mercury can reduce the surface tension of mercury and improve the wetting effect. Oil, other organic matter and fine mud will pollute the surface of gold particles. Sulfide, talc, graphite and arsenide in the ore are easy to adhere to the surface of mercury, which also affects the wettability of mercury to gold.
(5) Amount of mercury added in mercury mixing
When mixing mercury, the amount of mercury should be appropriate, too much will reduce the elasticity and consistency of mercury paste, so that the mercury paste will be lost with the pulp. The insufficient amount of mercury makes the mercury paste hard, lose its elasticity and reduce its gold capture performance. After the mercury plate is put into production, the initial amount of mercury is 15-30g / m3, and mercury is added after 6-12 hours. The amount of mercury added is 2-5 times of the gold content of the ore, and the consumption of mercury is usually 3-8g / T of ore.
(5) Mercury plate slope
The slope of mercury plate affects the velocity of slurry and mineral stratification. Generally, the slope of mercury plate is 7-9 degrees
(6) Temperature
Temperature should also affect the effect of mercury mixing. Too low temperature will increase the viscosity of mercury and affect the mixing effect. Too high temperature will increase the mobility of mercury, resulting in the loss of part of mercury gold with the loss of mercury. Therefore, the mercury mixing index is prone to seasonal changes, and the mercury mixing temperature should generally be greater than 15 degrees. The method of adding mercury and adjusting pulp concentration is adopted to eliminate the influence of temperature.